Stabilize ultra-cold molecular samples
Theoretical physicists have proposed a protocol using a laser beam to inhibit unwanted chemical reactions that destabilize ultra-cold molecular samples.
Ultra-cold gases, whose temperature is very close to absolute zero, are physical objects that reveal the most intriguing secrets of quantum matter. They are also systems of choice for applications in information or quantum simulation. These gases are composed of atoms, but also of diatomic molecules, whose electric dipole moment makes them more sensitive to electromagnetic fields, and therefore more easily manipulated. However, these increased possibilities of control are coupled with a greater complexity to obtain molecular samples which can be exploited for the applications mentioned above.
As such, the obtaining in several teams around the world of heteronuclear molecules in their absolute ground state (electronic, ro-vibrational and hyperfine) has been a major achievement of the last decade. These molecules are essentially composed of alkaline atoms such as sodium, potassium or even rubidium. However, once these samples were formed, the various experimental teams were forced to note their great instability. After a fraction of a second, the molecules disappear under the effect of undesirable chemical reactions, the exact nature of which is still the subject of various studies today.
In a recent article published in Physical Review Letters, theoretical physicists from the Aimé Cotton Laboratory (LAC, Orsay), the Carnot Interdisciplinary Laboratory in Burgundy (ICB, Dijon) and the Nuclear Research Institute (ATOMKI, Debrecen, Hungary) proposed a new protocol to increase the stability of ultra-cold molecular samples. Its principle is to prevent two molecules from approaching each other sufficiently to trigger an unwanted chemical reaction. Using a laser beam of carefully chosen frequency and intensity, it is possible to ensure that two molecules approach systematically like two magnets whose north poles face each other, and therefore which repel each other at the point of not being able to stick.
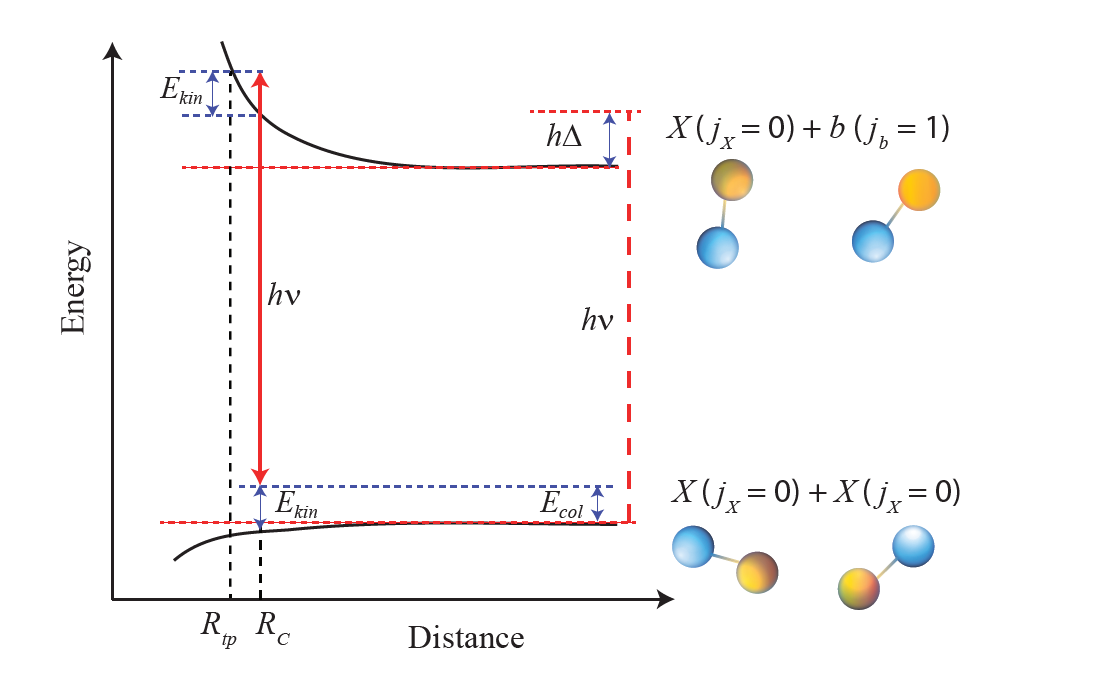
This screening effect between molecules has been demonstrated using a statistical electric field or microwave instead of the laser beam, the latter however proving to be easier to implement in the experiments. Its major drawback is that it induces fluorescence in the molecular gas, which has the effect of destroying the expected screening effect. Here the researchers got around this problem by choosing a laser frequency close to a transition to a metastable molecular level, which considerably limits the harmful effect of fluorescence.
In this theoretical work, the physicists first calculated the interaction energy between two molecules, in the presence of a laser beam whose frequency is close to a molecular transition. A laser frequency slightly higher than the transition frequency makes it possible to promote a repulsive interaction between molecules. But since the magnitude of this repulsion strongly depends on the frequency and intensity of the laser, the researchers determined the optimal values of these parameters, that is to say those which minimize the rate constant characterizing the reaction of destruction of molecules. This constant was calculated by numerically solving the Schrödinger equation, which describes the evolution of quantum systems.
Interaction energies and rate constants have been calculated in detail in the case of the sodium-rubidium (NaRb) molecule, on which the team of theorists has a fruitful collaboration with experimenters from Hong Kong. The simulations show that, within a range of experimentally accessible laser parameters, the rate constant of adverse reactions can be approximately divided by 1000, compared to the situation without a laser. The result is a significant increase in the stability of molecular samples which thus become exploitable for future experiments. Finally, estimates made for the other alkaline diatomic molecules show that the protocol presented in detail for NaRb is applicable for most of them.
- For more information : T. Xie, M. Lepers, R. Vexiau, A. Orban, O. Dulieu and N. Bouloufa-Maafa. Optical shielding of destructive chemical reactions between ultracold ground-state NaRb molecules, Phys. Rev. Lett, 125, 153202 (2020).
- kc_data:
- a:8:{i:0;s:0:"";s:4:"mode";s:2:"kc";s:3:"css";s:0:"";s:9:"max_width";s:0:"";s:7:"classes";s:0:"";s:9:"thumbnail";s:0:"";s:9:"collapsed";s:0:"";s:9:"optimized";s:0:"";}
- kc_raw_content:
