Discovery: A protein cocktail revolutionizes nanoparticle medicine
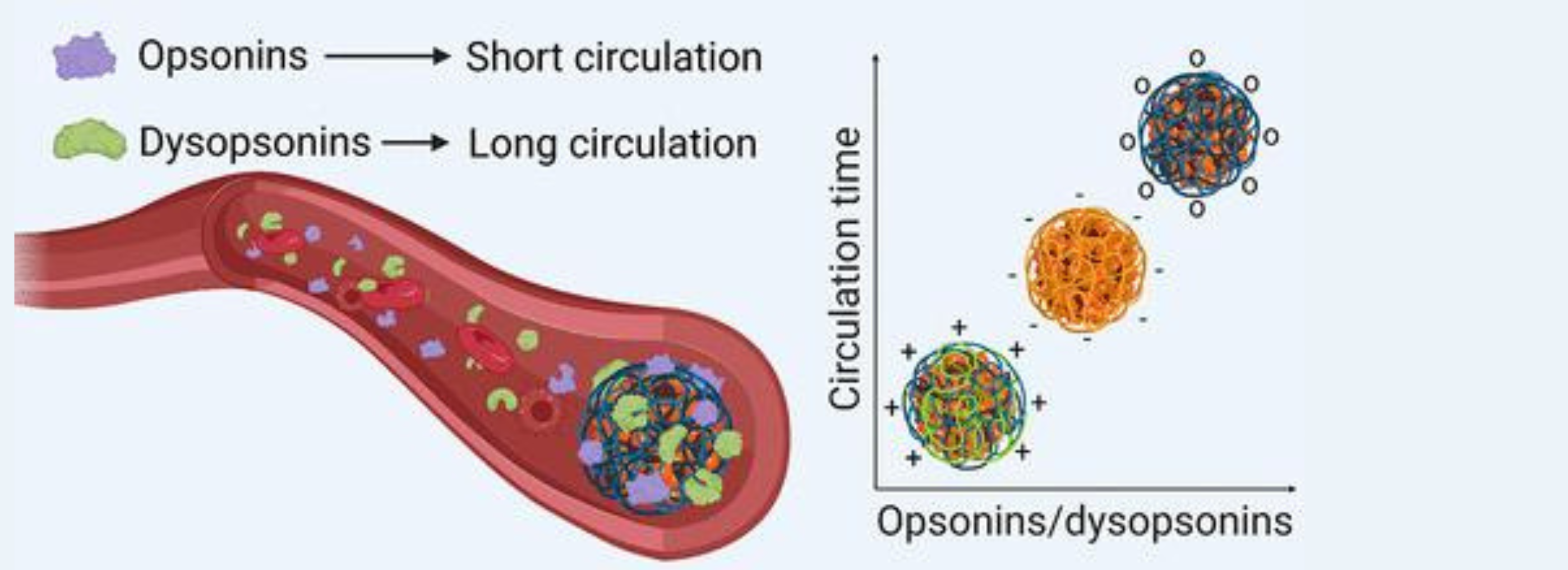
Nanoparticles, tiny structures with particular physicochemical properties, are revolutionizing the field of medicine. They are used in the diagnosis and targeting of various organs and pathologies, thereby reducing the need for high drug doses. However, a major obstacle hinders their effectiveness: the uncontrolled adsorption of biological proteins as they circulate in the human body.
Researchers from the Carnot Interdisciplinary Laboratory in Burgundy (ICB, CNRS/COMUE Université Bourgogne Franche-Comté), biologists from the George François Leclerc Center in Dijon (CGFL), pharmacists from the Biopharmaceutical Sciences group (University of Geneva, Switzerland) and chemists from the Department of Chemistry at the University of Notre Dame (Indiana, USA) and the Department of Radiology at Stanford University (California, USA) joined together to solve this problem. Their interdisciplinary and international study made it possible to discover a protein cocktail that significantly influences the biocirculation of nanoparticles, thus paving the way for improved medical treatments.
As nanoparticles circulate in biological fluids, they interact with the proteins present, which can reduce their effectiveness and slow down their targeting ability. Although proteins from the opsonin and dysopsonin family have been identified to influence the circulation of nanoparticles by making them more or less visible to the immune system, the researchers found that there remained many unknowns regarding the precise characterization of the proteins. responsible for this phenomenon.
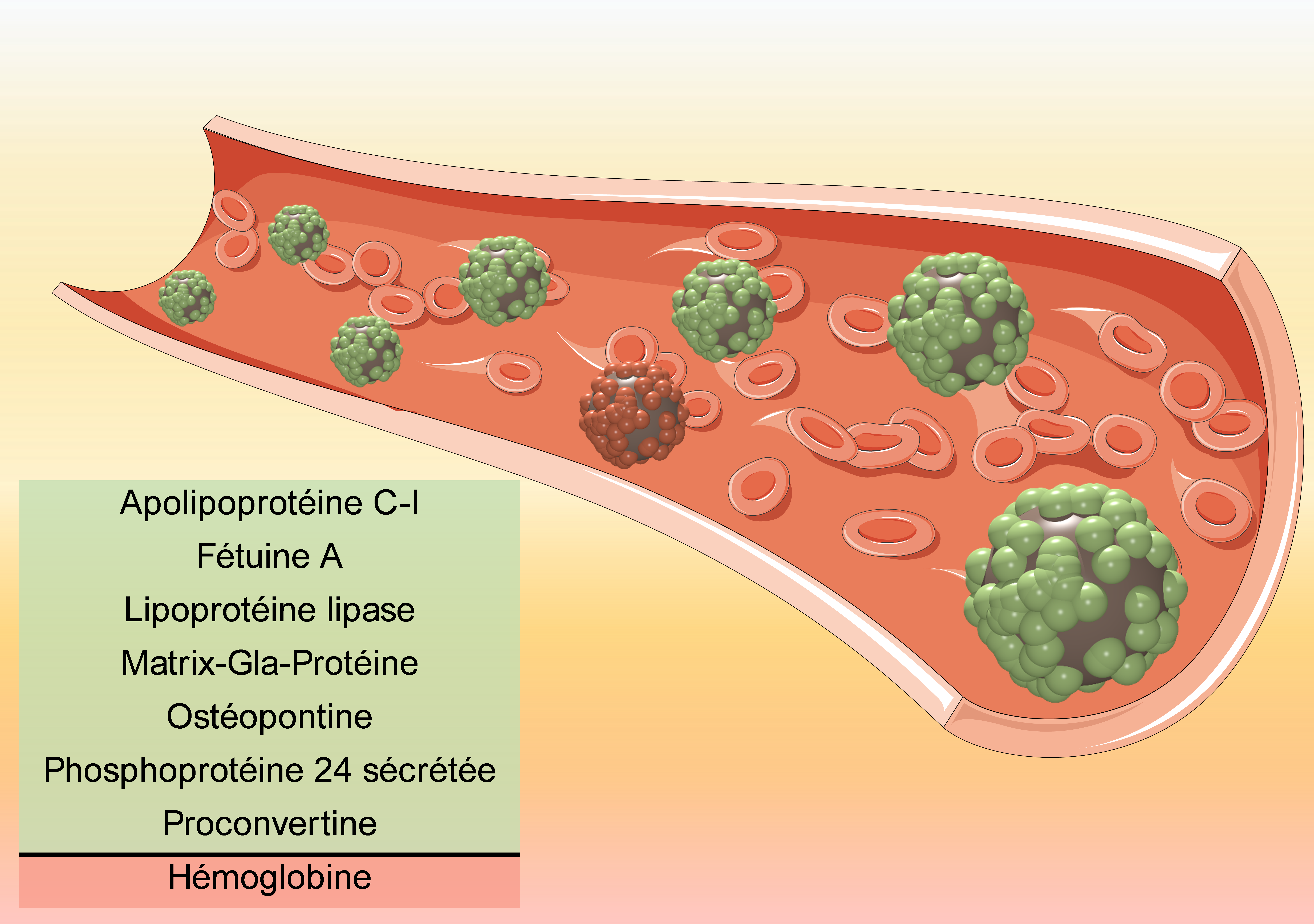
Figure: Seven proteins (in green) increase the blood circulation time of NPs and one decreases it (in red) © Maurizi et al. and Servier Medical Art.
- To find out more: Identification of the Proteins Determining the Blood Circulation Time of Nanoparticles Cintia Marques*, Mohammad Javad Hajipour*, Célia Marets, Alexandra Oudot, Reihaneh Safavi-sohi, Mélanie Guillemin, Gerrit Borchard, Olivier Jordan, Lucien Saviot, and Lionel Maurizi*, 2023, 17, 13, 12458–12470, June 28, 2023, https://doi.org/10.1021/acsnano.3c02041
- Read the article published on the INP (CNRS)
The study consisted of injecting nanoparticles functionalized with polymers of different sizes and carrying various charges into rats. The results revealed that the length of the polymer did not have a significant impact on the flow of nanoparticles, while the charge played a determining role. Positively charged nanoparticles had a very short plasma retention time (less than 5 minutes), negatively charged ones circulated for 30 minutes, and neutral nanoparticles were detected for 90 minutes.
Additionally, the researchers found that the ratios of opsonins to dysopsonins on the nanoparticles were not significantly different despite variations in circulation time and surface chemistry. They identified twenty-four proteins responsible for the behavior of the nanoparticles, seven of which extended their blood circulation time. Furthermore, the presence of hemoglobin was associated with a reduction in the circulation of nanoparticles.
These revolutionary discoveries open the way to a better understanding of the biological behaviors of nanoparticles, essential for their development in nanomedicine, as well as to anticipate their toxicity after injection or accidental exposure. The proteins identified in this study could be used to functionalize the surface of nanoparticles, thus improving their ability to specifically target areas to be treated in therapy or diagnosis. These promising results are published in the journal ACS Nano, offering an exciting glimpse into the future of medicine through nanoparticle science.
- kc_data:
- a:8:{i:0;s:0:"";s:4:"mode";s:2:"kc";s:3:"css";s:0:"";s:9:"max_width";s:0:"";s:7:"classes";s:0:"";s:9:"thumbnail";s:0:"";s:9:"collapsed";s:0:"";s:9:"optimized";s:0:"";}
- kc_raw_content:
- [kc_row use_container="yes" _id="167696"][kc_column width="12/12" video_mute="no" _id="986303"][kc_column_text _id="420506"]
Nanoparticles, tiny structures with particular physicochemical properties, are revolutionizing the field of medicine. They are used in the diagnosis and targeting of various organs and pathologies, thereby reducing the need for high drug doses. However, a major obstacle hinders their effectiveness: the uncontrolled adsorption of biological proteins as they circulate in the human body.
Researchers from the Carnot Interdisciplinary Laboratory in Burgundy (ICB, CNRS/COMUE Université Bourgogne Franche-Comté), biologists from the George François Leclerc Center in Dijon (CGFL), pharmacists from the Biopharmaceutical Sciences group (University of Geneva, Switzerland) and chemists from the Department of Chemistry at the University of Notre Dame (Indiana, USA) and the Department of Radiology at Stanford University (California, USA) joined together to solve this problem. Their interdisciplinary and international study made it possible to discover a protein cocktail that significantly influences the biocirculation of nanoparticles, thus paving the way for improved medical treatments.
As nanoparticles circulate in biological fluids, they interact with the proteins present, which can reduce their effectiveness and slow down their targeting ability. Although proteins from the opsonin and dysopsonin family have been identified to influence the circulation of nanoparticles by making them more or less visible to the immune system, the researchers found that there remained many unknowns regarding the precise characterization of the proteins. responsible for this phenomenon.
[/kc_column_text][kc_spacing height="30px" _id="630713"][/kc_column][/kc_row][kc_row use_container="yes" _id="66031"][kc_column width="45%" _id="791835"][kc_single_image image_size="full" _id="401797" image_source="media_library" image="43733"][kc_spacing height="10px" _id="426734"][kc_column_text _id="936101"]Figure: Seven proteins (in green) increase the blood circulation time of NPs and one decreases it (in red) © Maurizi et al. and Servier Medical Art.
[/kc_column_text][kc_spacing height="20" _id="590760"][kc_column_text _id="863926"]- To find out more: Identification of the Proteins Determining the Blood Circulation Time of Nanoparticles Cintia Marques*, Mohammad Javad Hajipour*, Célia Marets, Alexandra Oudot, Reihaneh Safavi-sohi, Mélanie Guillemin, Gerrit Borchard, Olivier Jordan, Lucien Saviot, and Lionel Maurizi*, 2023, 17, 13, 12458–12470, June 28, 2023, https://doi.org/10.1021/acsnano.3c02041
- Read the article published on the INP (CNRS)
The study consisted of injecting nanoparticles functionalized with polymers of different sizes and carrying various charges into rats. The results revealed that the length of the polymer did not have a significant impact on the flow of nanoparticles, while the charge played a determining role. Positively charged nanoparticles had a very short plasma retention time (less than 5 minutes), negatively charged ones circulated for 30 minutes, and neutral nanoparticles were detected for 90 minutes.
Additionally, the researchers found that the ratios of opsonins to dysopsonins on the nanoparticles were not significantly different despite variations in circulation time and surface chemistry. They identified twenty-four proteins responsible for the behavior of the nanoparticles, seven of which extended their blood circulation time. Furthermore, the presence of hemoglobin was associated with a reduction in the circulation of nanoparticles.
These revolutionary discoveries open the way to a better understanding of the biological behaviors of nanoparticles, essential for their development in nanomedicine, as well as to anticipate their toxicity after injection or accidental exposure. The proteins identified in this study could be used to functionalize the surface of nanoparticles, thus improving their ability to specifically target areas to be treated in therapy or diagnosis. These promising results are published in the journal ACS Nano, offering an exciting glimpse into the future of medicine through nanoparticle science.
[/kc_column_text][/kc_column][/kc_row]
