Molecules, Atoms, Reactivity and Scattering (MARS)
Le développement d'outils théoriques et informatiques pour la modélisation des systèmes moléculaires à symétrie élevée constitue un savoir faire spécifique reconnu de la communauté scientifique.
Les études actuelles portent sur l'élaboration de modèles pour la description des interactions intramoléculaires, intermoléculaires et l'interaction molécule – rayonnement (états excités, processus collisionnels, processus multiphotoniques). Ces recherches sont sous-tendues par les applications à la physique de l'atmosphère, la planétologie et la photoexcitation laser. Les résultats d'analyse de spectres sont directement accessibles via les systèmes informatiques TDS développés au Laboratoire.
Un second axe de recherche de l'équipe concerne l'étude théorique de processus collisionnels d'intérêt astrophysique et atmosphérique.
Citons par exemple le traitement complet des collisions réactives du type A + BC → AB + C qui va de la détermination de la surface d'énergie potentielle électronique (calculs ab initio et ajustements) aux calculs des sections efficaces et constantes de vitesse (dynamique quantique à l'aide d'un formalisme indépendant du temps basé sur les coordonnées hypersphériques).
Vincent Boudon
Responsable
Vincent.Boudon@u-bourgogne.fr
Tél : 03 80 39 59 17
MEMBRES

Thématiques de recherche
Méthane et planétologie
Calculs ab initio pour la dynamique et la spectroscopie moléculaire
Dynamique quantique réactionnelle
Analyses de spectres moléculaires et applications aux atmosphères
Gestion et valorisation des données de la recherche
- kc_data:
- a:8:{i:0;s:0:"";s:4:"mode";s:2:"kc";s:3:"css";s:0:"";s:9:"max_width";s:0:"";s:7:"classes";s:0:"";s:9:"thumbnail";s:0:"";s:9:"collapsed";s:0:"";s:9:"optimized";s:0:"";}
- kc_raw_content:
- [kc_row use_container="yes" _id="861549"][kc_column width="55%" _id="265309"][kc_column_text _id="992260"]
Molecules, Atoms, Reactivity and Scattering (MARS)
Le développement d'outils théoriques et informatiques pour la modélisation des systèmes moléculaires à symétrie élevée constitue un savoir faire spécifique reconnu de la communauté scientifique.
Les études actuelles portent sur l'élaboration de modèles pour la description des interactions intramoléculaires, intermoléculaires et l'interaction molécule - rayonnement (états excités, processus collisionnels, processus multiphotoniques). Ces recherches sont sous-tendues par les applications à la physique de l'atmosphère, la planétologie et la photoexcitation laser. Les résultats d'analyse de spectres sont directement accessibles via les systèmes informatiques TDS développés au Laboratoire.
Un second axe de recherche de l'équipe concerne l'étude théorique de processus collisionnels d'intérêt astrophysique et atmosphérique.
Citons par exemple le traitement complet des collisions réactives du type A + BC → AB + C qui va de la détermination de la surface d'énergie potentielle électronique (calculs ab initio et ajustements) aux calculs des sections efficaces et constantes de vitesse (dynamique quantique à l'aide d'un formalisme indépendant du temps basé sur les coordonnées hypersphériques).
[/kc_column_text][/kc_column][kc_column width="5%" _id="348190"][/kc_column][kc_column width="40%" _id="657220"][kc_column_text _id="564775"]Vincent Boudon
Responsable
Vincent.Boudon@u-bourgogne.fr
[/kc_column_text][kc_spacing height="30px" _id="567226"][kc_column_text _id="692307"]
Tél : 03 80 39 59 17MEMBRES
[/kc_column_text][kc_spacing height="30px" _id="987914"][kc_single_image image_size="141x100" _id="37651" image_source="media_library" image="42334"][kc_spacing height="11px" _id="157977"][kc_column_text _id="263174"]{slide=Permanents}
- BOUDON Vincent, DR CNRS
- GABARD Tony, M. de Conférences
- GUILLON Grégoire, M. de Conférences
- HONVAULT Pascal, Professeur
- LEPERS Maxence, CR CNRS
- LEROY Claude, Professeur
- LOETE Michel, Professeur émérite
- RICHARD Cyril, IR CNRS
{/slide}
{slide=Non-permanents}
- HARDY Pierre, doctorant
- HOVHANNESYAN Gohar, doctorante
- MERKULOVA Mariia, doctorante
- MOMIER Rodolphe, doctorant{/slide}
{slide=Anciens membres}
- BUSSERY-HONVAULT Béatrice, DR CNRS (retraitée)
- DUPRÉ Patrick, CR CRNS (retraité)
- CHAMPION Jean-Paul, Professeur (retraité)
- MICHELOT Françoise, Professeur (retraitée)
- WENGER Christian, IR CNRS (retraité depuis janvier 2014)
{/slide}
[/kc_column_text][/kc_column][/kc_row][kc_row use_container="yes" _id="291698" cols_gap="{`kc-css`:{}}" force="__empty__" animate="fadeInLeft||"][kc_column width="12/12" video_mute="no" _id="48345"][kc_spacing height="50px" _id="349265"][kc_column_text _id="516251"]Thématiques de recherche
[/kc_column_text][kc_spacing height="30px" _id="632976"][/kc_column][/kc_row][kc_row use_container="yes" _id="898298" cols_gap="{`kc-css`:{}}" force="__empty__" animate="fadeInLeft||"][kc_column width="2%" video_mute="no" _id="787947"][/kc_column][kc_column width="45%" video_mute="no" _id="41533"][kc_spacing height="20" _id="147839"][kc_column_text _id="831711"]Méthane et planétologie
[/kc_column_text][kc_spacing height="20" _id="44084"][kc_column_text _id="167388"]{slide=Modeling the absorption spectrum of methane}
For applications to Earth's, planetary and exo-planetary atmospheres.
Methane (CH4), the simplest stable hydrocarbon molecule, is a species of primary importance for many domains:
- It is the third major greenhouse gas (after water and carbon dioxide) in the Earth's atmosphere.
- It is an important constituent of some planetary atmospheres like the giant planets (Jupiter, Saturn, Uranus, Neptune) and Titan, Pluto, … , as well as of the atmospheres of brown dwarfs, of some "cold" stars, etc.
- It has been recently discovered in the atmosphere of exoplanets of "hot-jupiter" type like HD189733b or HD209458b.
- It plays an important role in combustion processes.
Although it is a small and simple molecule, its rovibrational spectroscopy is very complicated. This is mainly due to the high symmetry of this tetrahedral system (which leads to the existence of many degeneracies) and to its intricated vibrational structure.
As a matter of fact, the four fundamental vibration frequencies of CH4 (three of then being degenerate oscillators) obey a simple approximate relation (see Table 1), which leads to a specific vibrational structure with vibrational levels grouped into "packets" called polyads.
[caption id="" align="aligncenter" width="542"]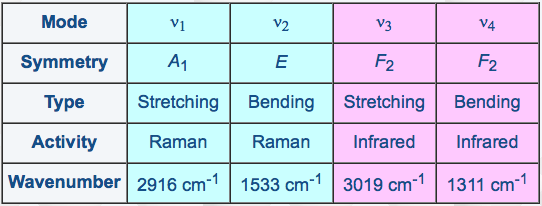 Table 1: The normal modes of methane[/caption]
Table 1: The normal modes of methane[/caption]
Figure 1 shows the first ten polyads of methane. It is clear that the complexity of the spectrum increases rapidly with energy, due to the increasing number of levels and sublevels.
[caption id="" align="aligncenter" width="800"]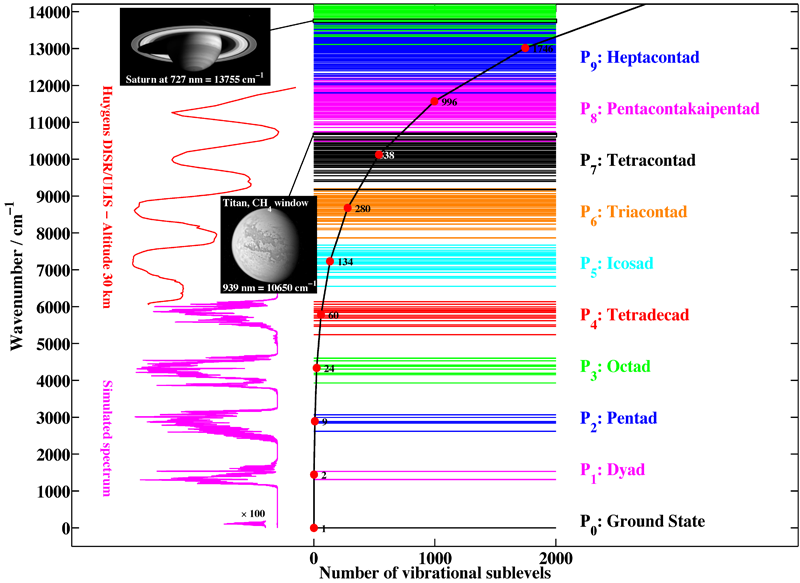 Figure 1: The polyads of methane, illustrating the complexity of this spectrum. Horizontal lines represent vibration energy levels. The black curve gives the number of vibrational sublevels for each polyad. The names correspond to the different absorption bands. Different spectral regions are illustrated by images and spectra: in pink, a simulated spectrum for lower polyads and, in red, an example of the spectra recorded on Titan by the Huygens probe (JPL images PIA05381 and PIA06220 – Credit: NASA/JPL/Space Science Institute).[/caption]
Figure 1: The polyads of methane, illustrating the complexity of this spectrum. Horizontal lines represent vibration energy levels. The black curve gives the number of vibrational sublevels for each polyad. The names correspond to the different absorption bands. Different spectral regions are illustrated by images and spectra: in pink, a simulated spectrum for lower polyads and, in red, an example of the spectra recorded on Titan by the Huygens probe (JPL images PIA05381 and PIA06220 – Credit: NASA/JPL/Space Science Institute).[/caption]
Our group is the world leader concerning the analysis of high-resolution spectra (infrared absorption and Raman scattering) of methane. Needless to say, however, that the whole wavenumber range displayed in Figure 1 is far from being understood. At the present time, the octad is the highest polyad which is fully understood (a global fit of the 0 to 4800 cm-1 region for both positions and intensities has been performed), the analysis of the tetradecad being still partial, but in good progress and already convincing (see Figure 4 below)). Figure 2 shows an exemple of a simulation of the infrared absorption spectrum of methane.
[caption id="" align="aligncenter" width="800"]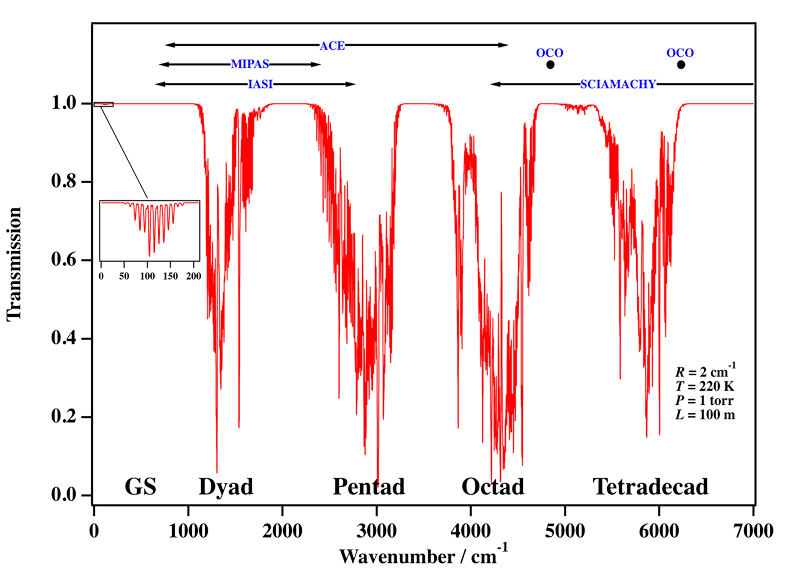 Figure 2: Calculated infrared absorption of methane. GS is the ground vibrational state. Some Earth-observing satellite/instrument wavenumber ranges are indicated at the top.[/caption]
Figure 2: Calculated infrared absorption of methane. GS is the ground vibrational state. Some Earth-observing satellite/instrument wavenumber ranges are indicated at the top.[/caption]
Figure 3 illustrates the complexity of the level mixings in the excited polyads of methane. The example shown is that of the reduced energy levels in the octad, as a function of the rotational quantum number J. The colors show, for each rovibrational level, the contribution of the different normal mode vibrations after diagonalization of the effective Hamiltonian.
[caption id="" align="aligncenter" width="800"]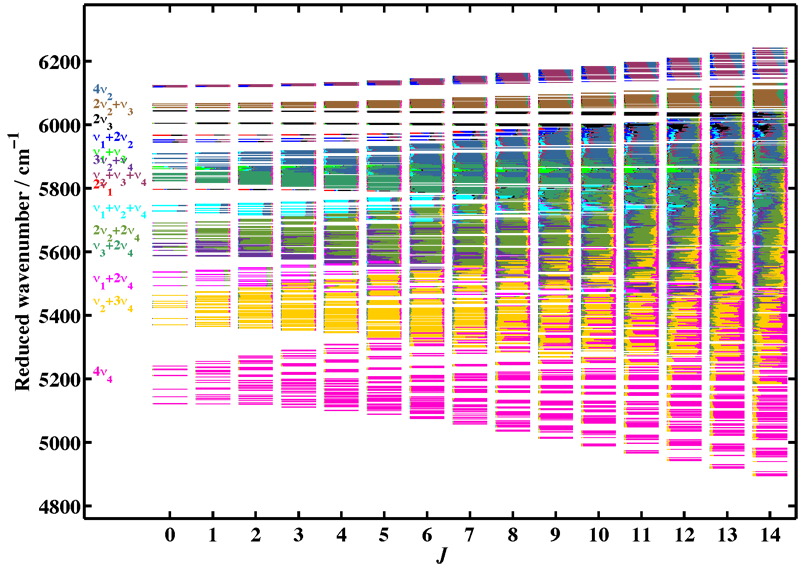 Figure 3: Level mixings in the Tetradecad of methane. Rovibrational levels are plotted a a function of the rotational quantum number J. The colors corresponds to the 14 different interacting vibrational levels.[/caption]
Figure 3: Level mixings in the Tetradecad of methane. Rovibrational levels are plotted a a function of the rotational quantum number J. The colors corresponds to the 14 different interacting vibrational levels.[/caption]
Figure 4 shows a recent comparison between an experimental and an simulated spectrum, in the so-called Tetradecad region. See the following paper doe more details:
[caption id="" align="aligncenter" width="800"]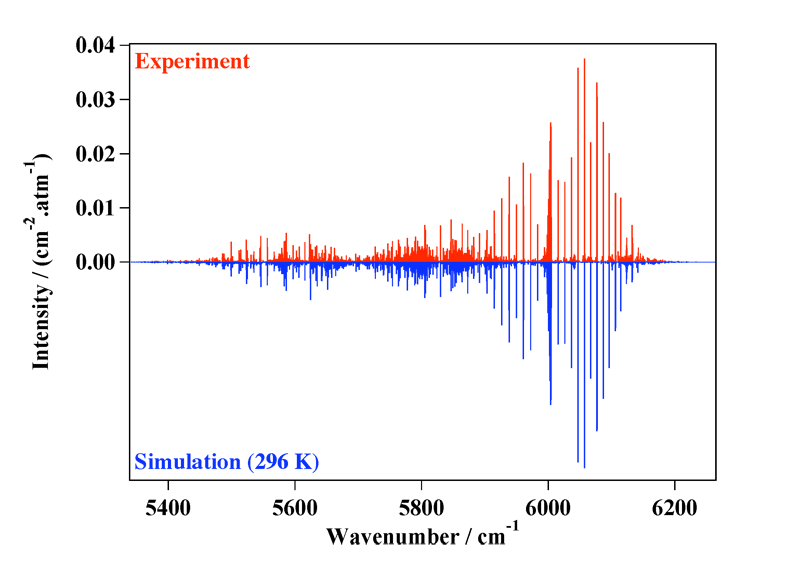 Figure 4: The Tetradecad region of methane at room remperature, compared to the simulation.[/caption]
Figure 4: The Tetradecad region of methane at room remperature, compared to the simulation.[/caption]
All our simulations and analyses are performed thanks to the group theoretical and tensorial tools developed in our group since many years for the spectroscopy of spherical tops, which is implemented in the STDS software.
We have started in 2013 the study of hot methane emission spectra, in collaboration with the Institut de Physique de Rennes (Prof. Robert Georges) and the AILES BEAMLINE of the Soleil Synchotron (Dr. Olivier Pirali). Figure 5 shows an example of such an emission spectrum in the bending Dyad region.
[caption id="" align="aligncenter" width="800"]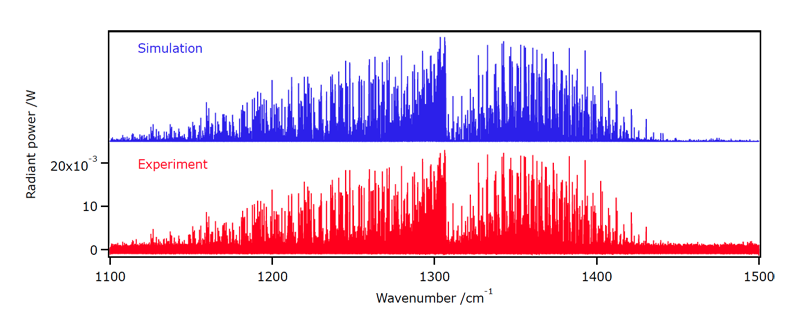 Figure 5: Emission spectrum of methane at ca. 1173 K, compared to the simulation.[/caption]
Figure 5: Emission spectrum of methane at ca. 1173 K, compared to the simulation.[/caption]
The 13CH4, 12CD4 and 12CH3D isotopologues are also studied. In the case of 12CH3D, the MIRS software is used.
Our results are widely used for atmospheric and planetary applications. Some references illustrating recent applications to the case of Titan's atmosphere can be found on the CH4@Titan ANR (2009-2012) page.
See also the following reference:
To calculate the methane partition function: click here.
Our calculated line lists can be downloaded for a database build up for the VAMDC (Virtual Atomic and Molecular Data Centre) consortium :
These linelists are also used to parly update the HITRAN database. See the following reference:
- Methane Line Parameters in the HITRAN Database. L. R. Brown, et al.,
Journal of Quantitative Spectroscopy and Radiative Transfer, 130, 201–219 (2013).
A special issue of the Journal of Molecular Spectroscopy dedicated to "Methane spectroscopy and its applications to planetary atmosphere including Earth's" has been published in 2013, with Athena Coustenis and Vincent Boudon as guest editors.
- Voir aussi : ANR e-PYTHEAS (2017–2020)
{/slide}
[/kc_column_text][kc_spacing height="30px" _id="43246"][kc_column_text _id="212183"]Calculs ab initio pour la dynamique et la spectroscopie moléculaire
[/kc_column_text][kc_spacing height="20" _id="56152"][kc_column_text _id="88782"]{slide=Plus d'informations}
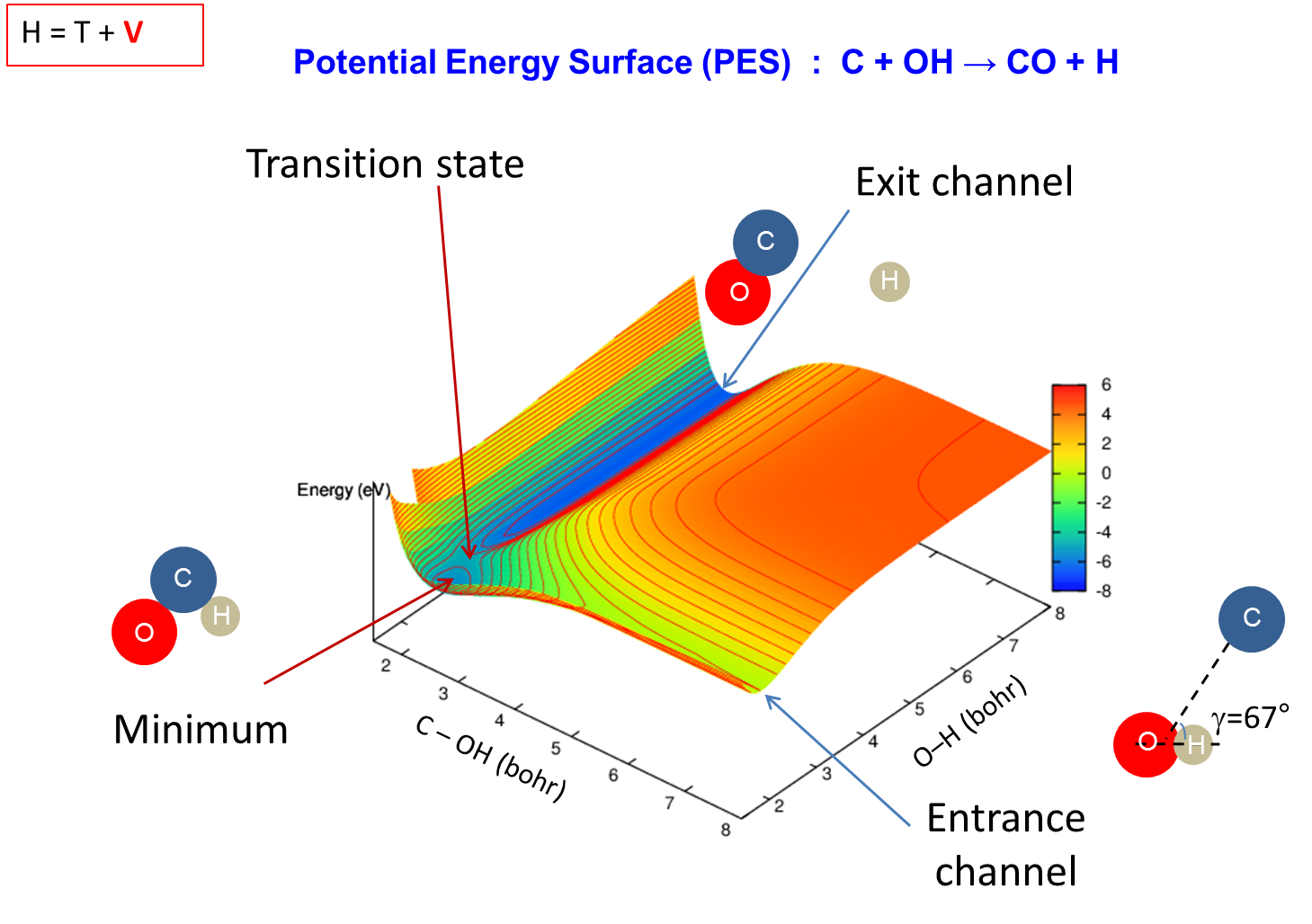
{/slide}
[/kc_column_text][kc_spacing height="30px" _id="621273"][kc_column_text _id="457764"]Dynamique quantique réactionnelle
[/kc_column_text][kc_spacing height="20" _id="430750"][kc_column_text _id="269921"]{slide=Plus d'informations}
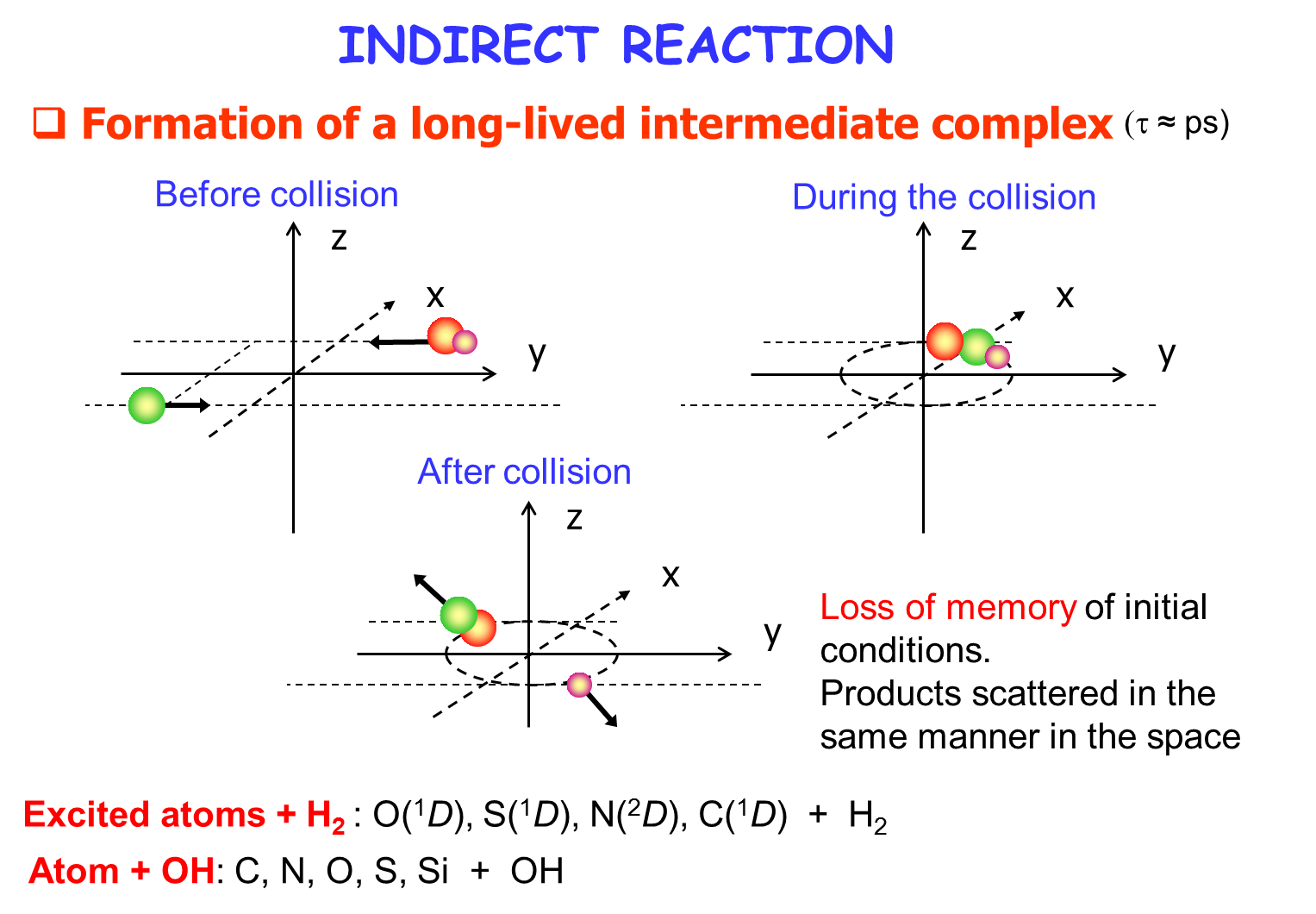
{/slide}
[/kc_column_text][kc_spacing height="20" _id="497482"][/kc_column][kc_column width="6%" video_mute="no" _id="2254"][/kc_column][kc_column width="45%" video_mute="no" _id="706574"][kc_spacing height="20" _id="501505"][kc_column_text _id="471466"]Analyses de spectres moléculaires et applications aux atmosphères
[/kc_column_text][kc_spacing height="20" _id="976046"][kc_column_text _id="991822"]{slide=Quelques exemples de spectres analysés}
- Sulfur Hexafluoride, SF6
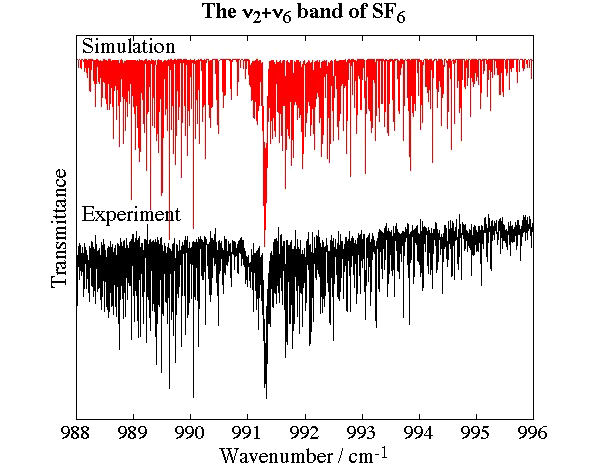
Experimental spectrum from M. Hepp and M. Herman (Lab. Chimie Physique Moléculaire, ULB, Brussels, Belgium).
References:
- High-Resolution Jet-Cooled Spectroscopy of SF6 : The ν2+ν6 Combination Band of 32SF6 and the ν3 band of the rare isotopomers, V. Boudon, M. Hepp, M. Herman, I. Pak and G. Pierre, Journal of Molecular Spectroscopy, 192, 359-367 (1998).
- Simultaneous Analysis of the ν2 Raman and ν2+ν6 Infrared Spectra of the SF6 Molecule, D. Bermejo, R. Z. Martínez, E. Loubignac, V. Boudon and G. Pierre, Journal of Molecular Spectroscopy, 201, 164-171 (2000).
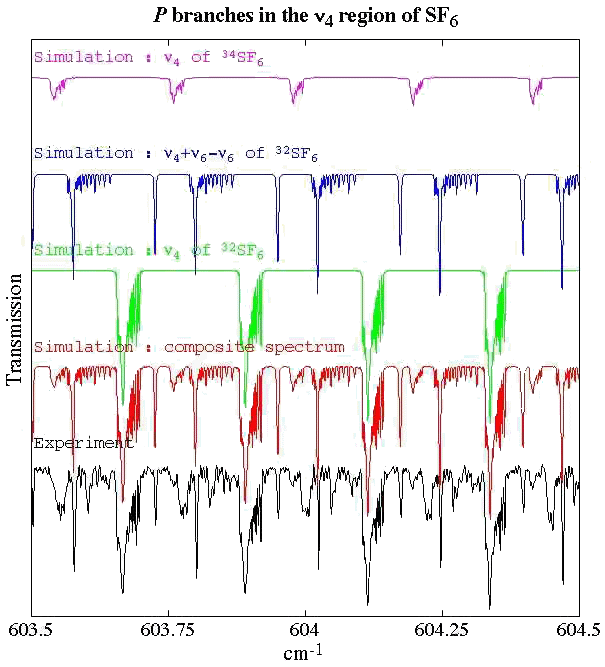
Experimental spectrum from H. Bürger (Anorganische Chemie, Universität-Gesamthochschule, Wuppertal, Germany).
Reference:
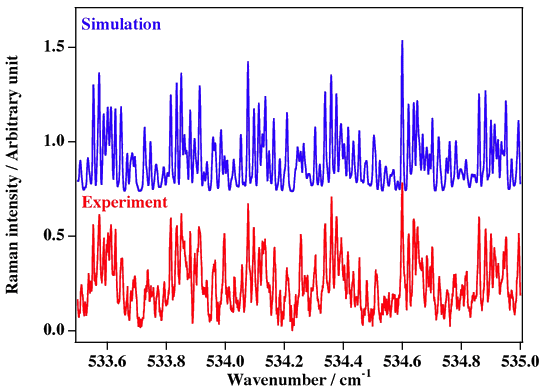
Experimental spectrum from J. L. Doménech, A. Ramos and D. Bermejo (Instituto de Estructura de la Materia, CSIC, Madrid, Spain).
Reference:
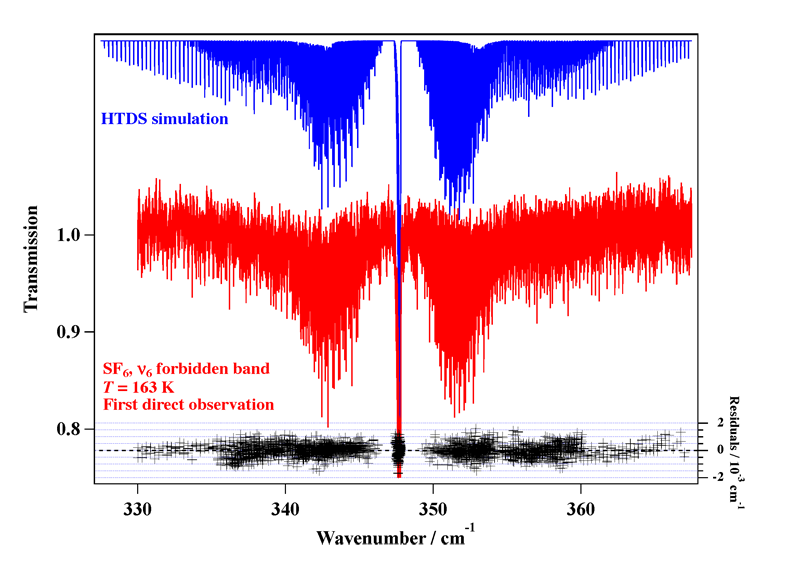
Experimental spectrum recorded at the AILES beamline of the SOLEIL Synchrotron (France).
Reference:
- Osmium Tetroxide, OsO4
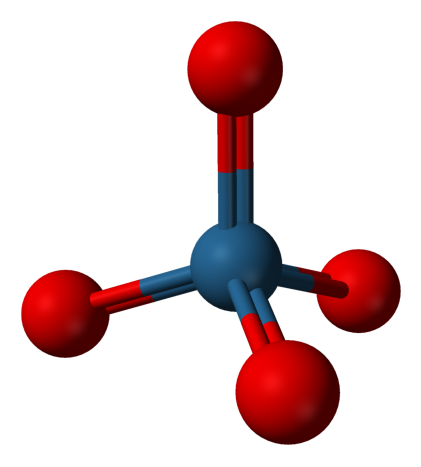
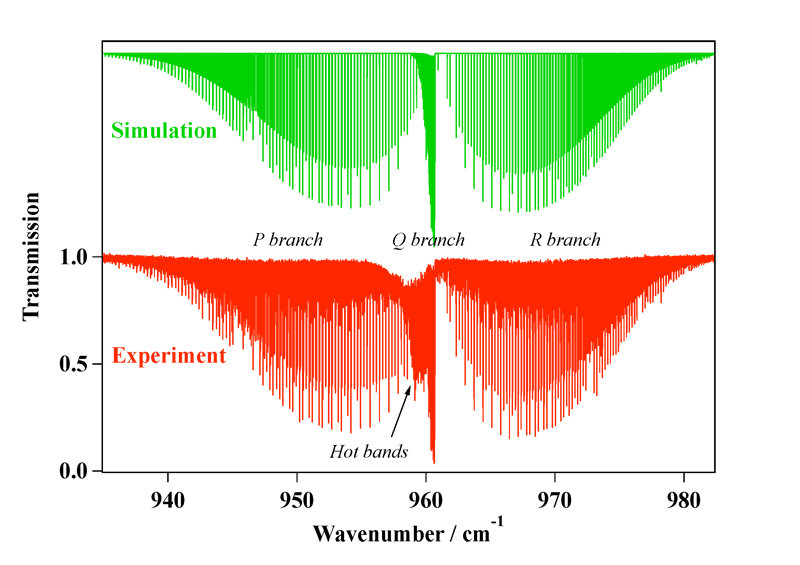
Experimental spectrum recorded at the AILES beamline of the SOLEIL Synchrotron (France).
References:
- High-Resolution Spectroscopy and Analysis of the ν1/ν3 Stretching Dyad of Osmium Tetroxide, M. Louviot, V. Boudon, L. Manceron, P. Roy and D. Balcon,
Journal of Quantitative Spectroscopy and Radiative Transfer, 113, 119–127 (2012). - High-Resolution Spectroscopy and Structure of Osmium Tetroxide. A Benchmark Study on 192OsO4, M. Louviot , V. Boudon, L. Manceron, D. Bermejo and R. Z. Martínez, Inorganic Chemistry, 51, 10356–10365 (2012).
- High-Resolution Stimulated Raman Spectroscopy and Analysis of the ν1 Band of OsO4, M. Louviot , V. Boudon, D. Bermejo, R. Z. Martínez and L. Manceron,
Journal of Raman Spectroscopy, 44, 63–69 (2013).
- Adamantane, C10H16
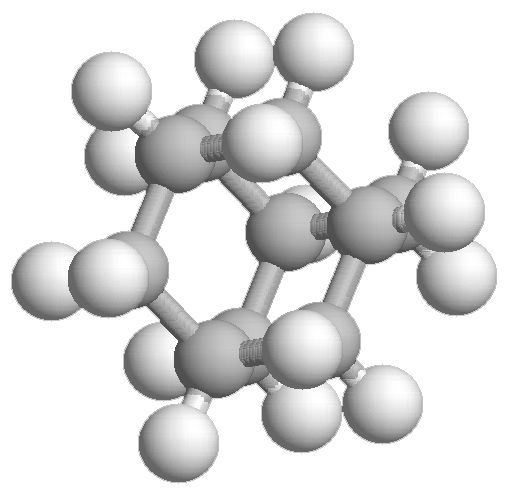
Experimental spectrum recorded at the AILES beamline of the SOLEIL Synchrotron (France).
Reference:
- Hexamethylenetetramine (HMT), C6N4H12


Experimental spectrum recorded at the AILES beamline of the SOLEIL Synchrotron (France).
Reference:
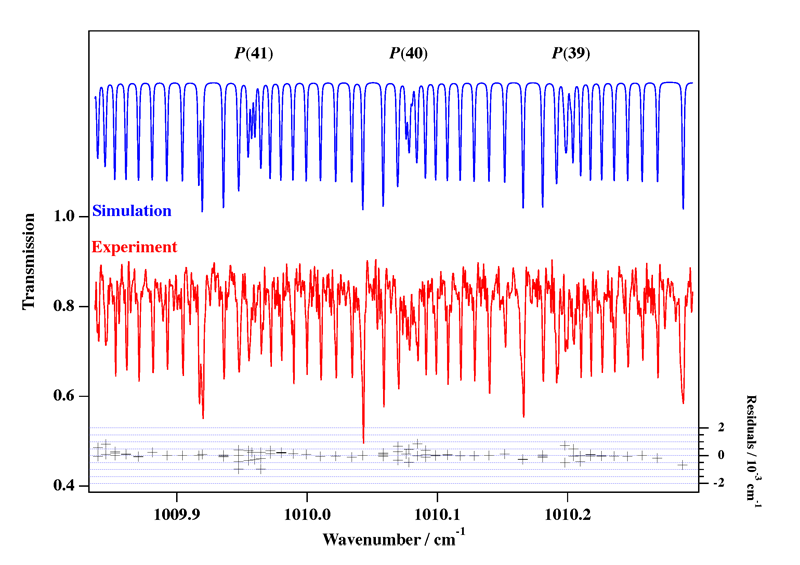
- White Phosphorus, P4
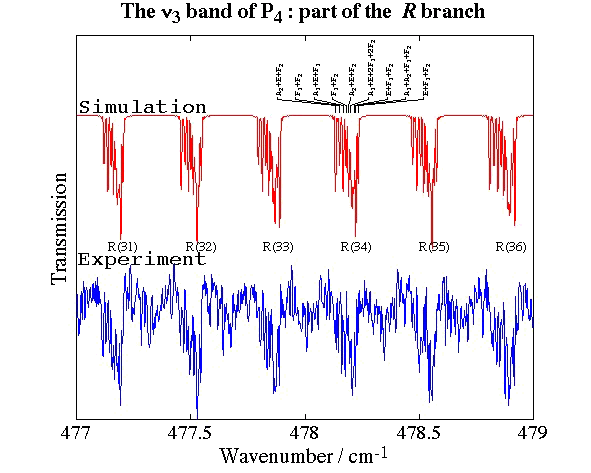
Experimental spectrum from E. B. Mkadmi and H. Bürger (Anorganische Chemie, Universität-Gesamthochschule, Wuppertal, Germany).
References:
- High-Resolution Fourier Transform Infrared Spectroscopy and Analysis of the ν3 Fundamental Band of P4, V. Boudon, E. B. Mkadmi, H. Bürger and G. Pierre,
Chemical Physics Letters, 305, 21-27 (1999). - Rotational-Vibrational Relative Equilibria and the Structure of Quantum Energy Spectrum of the Tetrahedral Molecule P4, Ch. Van-Hecke, D. A. Sadovskií, B. I. Zhilinskií and V. Boudon, European Physical Journal D, 17, 13-35 (2001).
- Deuterated Germane, GeD4
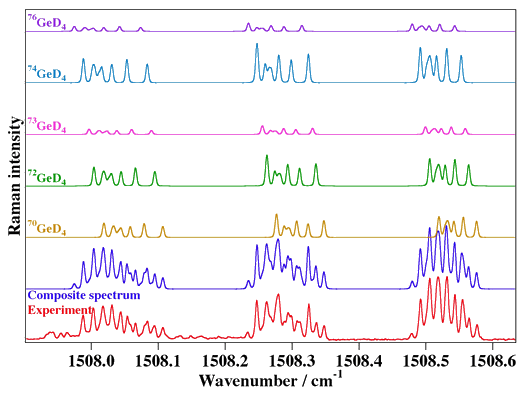
Experimental spectrum from R. Z. Martinez and D. Bermejo (Instituto de Estructura de la Materia, CSIC, Madrid, Spain).
Reference:
- Germanium Tetrafluoride, GeF4
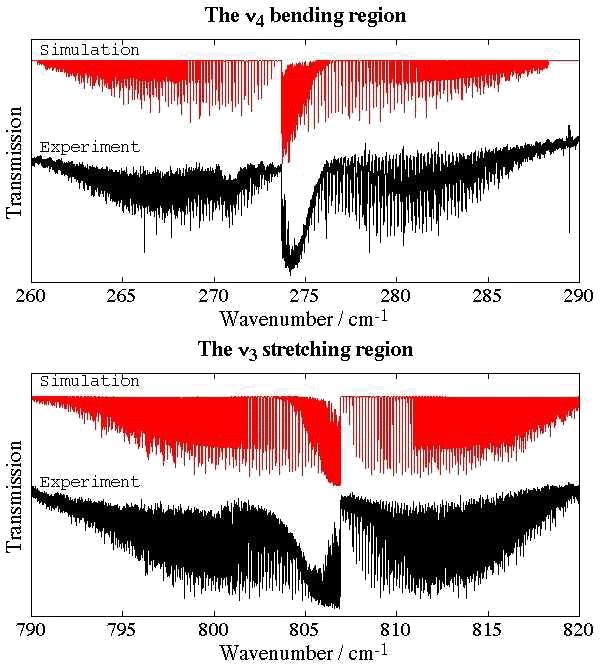
Experimental spectrum from H. Bürger and E. B. Mkadmi (Anorganische Chemie, Universität-Gesamthochschule, Wuppertal, Germany).
Reference:
- Selenium Hexafluoride, SeF6
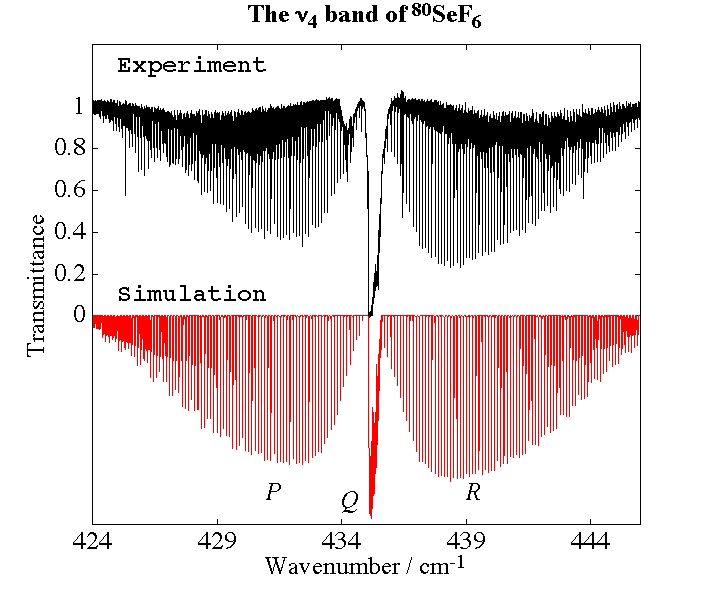
Experimental spectrum from H. Bürger (Anorganische Chemie, Universität-Gesamthochschule, Wuppertal, Germany).
References:
- Study of the v3=1 State of 80SeF6 by Fourier Transform Spectroscopy, M. Terki-Hassaine, G. Pierre, H. Bürger and H. Willner, Journal of Molecular Spectroscopy, 185, 93-97 (1997).
- High-Resolution Spectroscopy and Analysis of the ν4 band of 80SeF6, M. Rotger, V. Boudon, H. Bürger and H. Willner, Chemical Physics Letters, 339, 83-88 (2001).
- Molybdenum Hexacarbonyl, Mo(CO)6
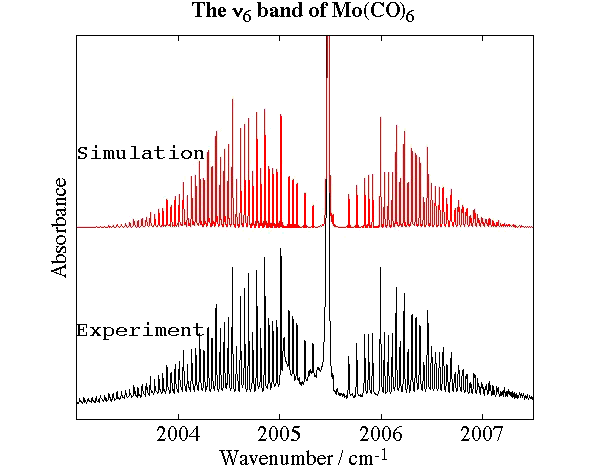
Experimental spectrum from P. Asselin, P. Soulard and L. Manceron (MONARIS, Paris, France).
References:
- High-Resolution Fourier Transform Infrared Spectroscopy and Analysis of the ν6 Band of Jet-Cooled Mo(CO)6, P. Asselin, P. Soulard, L. Manceron, V. Boudon and G. Pierre, Journal of Molecular Structure, 517, 145-155 (2000).
- Analysis of the "Unusual" Vibrational Components of Triply Degenerate Vibrational Mode ν6 of Mo(CO)6 Based on the Classical Interpretation of the Effective Rotation-Vibration Hamiltonian, G Dhont, D. Sadovskiì, B. Zhilinskiì and V. Boudon, Journal of Molecular Spectroscopy, 201, 95-108 (2000).
{/slide}
[/kc_column_text][kc_column_text _id="297123"]{slide=Spectroscopie dans un état électronique dégénéré}
The transition metal hexafluorides form a very interesting group of molecules, since they are the only compounds of these heavy elements with substantial vapor pressures at room temperature. This implies several industrial applications, such as isotope separation processes (especially with UF6, NpF6 and PuF6) or chemical vapor deposition of thin metal layers on a substrate (WF6, MoF6 for integrated circuits ; ReF6, IrF6 for anti-corrosion layers).
Among these molecules, there is a very interesting group which is traditionally called "colored hexafluorides". They are caracterized by the fact that they have an incomplete electronic subshell. This leads to low energy electronic levels, corresponding to transitions in the visible or near infrared regions (see figure below). Some of the "colored hexafluorides" also have the property of possessing an odd number of electrons (TcF6, RhF6, ReF6, IrF6, NpF6, AmF6).
[caption id="" align="aligncenter" width="542"]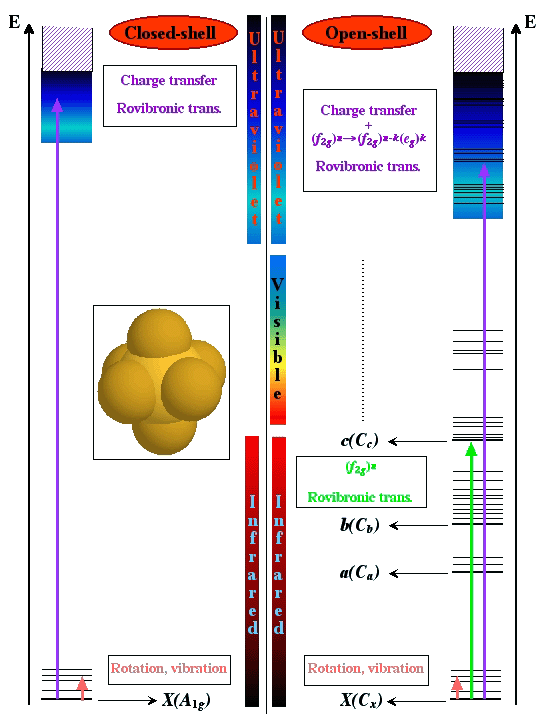 Difference between the absorption spectra of XY6 closed- and open-shell systems.[/caption]
Difference between the absorption spectra of XY6 closed- and open-shell systems.[/caption]
Their specificities of these molecules are that :
- They are open-shell systems, and their spectroscopy is similar to that of molecular ions.
- Their study is simpler than that of ions since it is easier to obtain a high density with molecules than with ions.
- They are very reactive (hygroscopic, ...).
- We have already recorded and analyzed low-resolution absorption and Raman spectra of IrF6, OsF6 and WF6 and obtained low-resolution absorption spectra of PtF6.
High-resolution spectra of WF6 and ReF6 using infrared spectroscopy (Fourier transform and diode laser) in a supersonic expansion jet were obtained in the Group of Professor Martin Quack (LPC-ETH Zürich). The analysis of the spectra of ReF6 requires a tensorial formalism adapted to rovibronic couplings and to the presence of half-integer angular momenta in octahedral symmetry. This work is still in progress
Transition-metal hexacarbonyls (Cr(CO)6, Mo(CO)6, W(CO)6, ...) are also interesting octahedral systems although this familly of molecules is much smaller that that of hexafluorides. There also exist an open-shell hexacarbonyl system with an odd number of electrons, namely V(CO)6 (vanadium hexacarbonyl).
Such studies could be extended to other open-shell systems like ReOF4 or to molecular ions or radicals of interest in astrophysics (CH3O, ...) or atmospherics physics.
[caption id="" align="aligncenter" width="694"]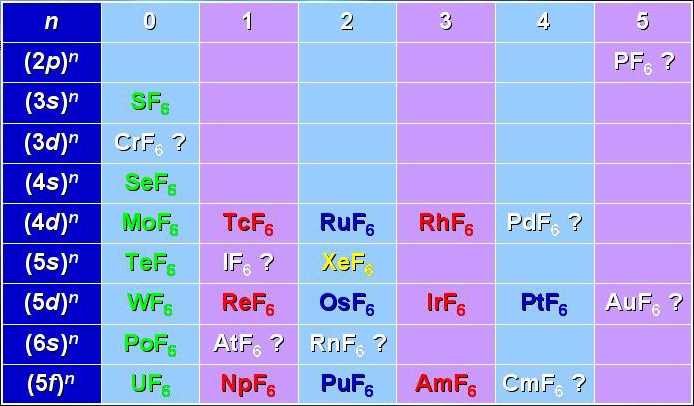 Table of the known and expected hexafluorides.[/caption]
Table of the known and expected hexafluorides.[/caption]
Example 1 : The NIR-Visible-UV absorption spectrum of some colored hexafluorides
[caption id="" align="aligncenter" width="714"]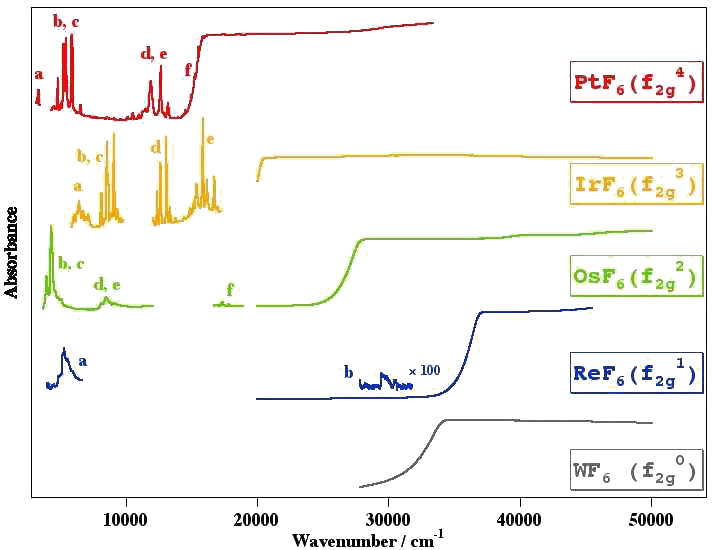 Spectra from M. Rotger and V. Boudon. The letters (a, b, c, ...) indicate the different electronic states.[/caption]
Spectra from M. Rotger and V. Boudon. The letters (a, b, c, ...) indicate the different electronic states.[/caption]
Example 2 : The NIR-Visible-UV absorption spectrum of IrF6
[caption id="" align="aligncenter" width="729"]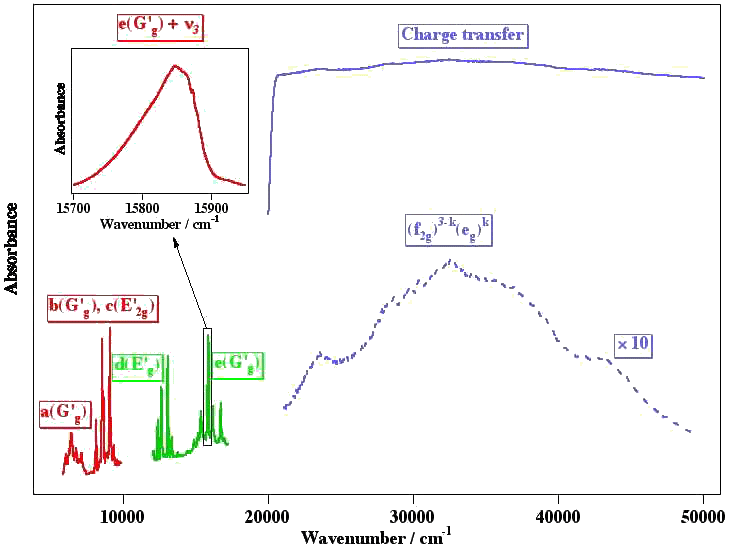 Spectra from M. Rotger and V. Boudon. The letters (a, b, c, ...) indicate the different electronic states (with symmetry in parentheses).[/caption]
Spectra from M. Rotger and V. Boudon. The letters (a, b, c, ...) indicate the different electronic states (with symmetry in parentheses).[/caption]
Réference:
Example 3 : Jet + diode laser high-resolution spectrum in the ν3 band of WF6
The case of a non-degenerate electronic ground state.
[caption id="" align="aligncenter" width="524"]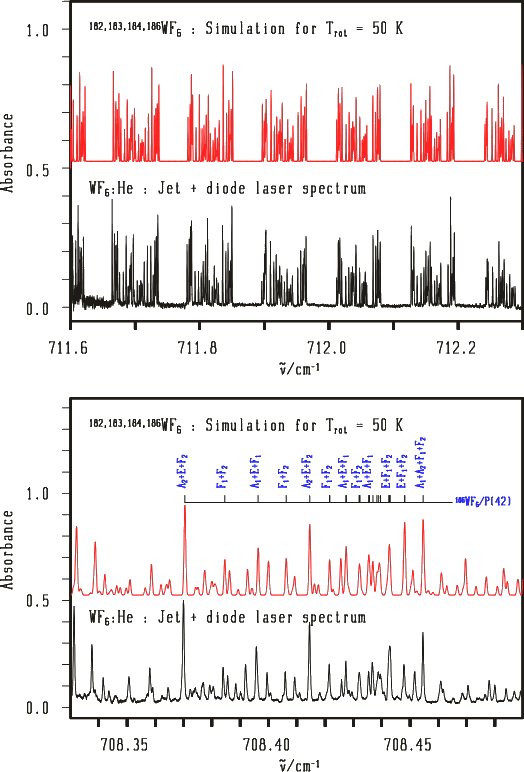 Spectra from V. Boudon, M. Rotger, Y. He, H. Hollenstein, M. Quack and U. Schmitt.[/caption]
Spectra from V. Boudon, M. Rotger, Y. He, H. Hollenstein, M. Quack and U. Schmitt.[/caption]
Reference:
Example 4 : Jet + FTIR and diode laser high-resolution spectrum in the ν3 band of ReF6
The case of a fourfold degenerate electronic ground state.
[caption id="" align="aligncenter" width="434"]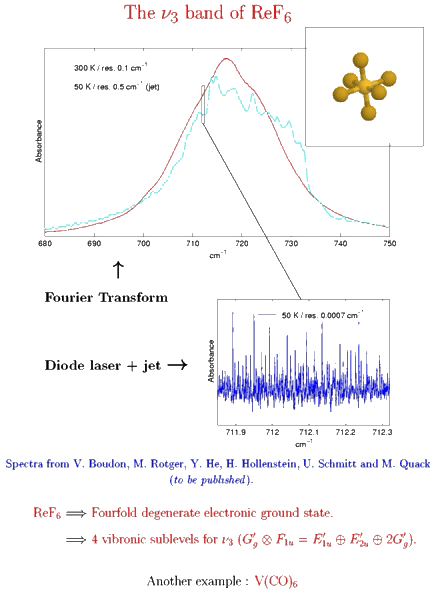 Spectra from V. Boudon, M. Rotger, Y. He, H. Hollenstein, M. Quack and U. Schmitt.[/caption]
Spectra from V. Boudon, M. Rotger, Y. He, H. Hollenstein, M. Quack and U. Schmitt.[/caption]
Reference:
Example 5 : Jet + FTIR spectrum in the ν6 band (C-O stretch) of V(CO)6
The case of a threefold degenerate electronic ground state.
[caption id="" align="aligncenter" width="574"]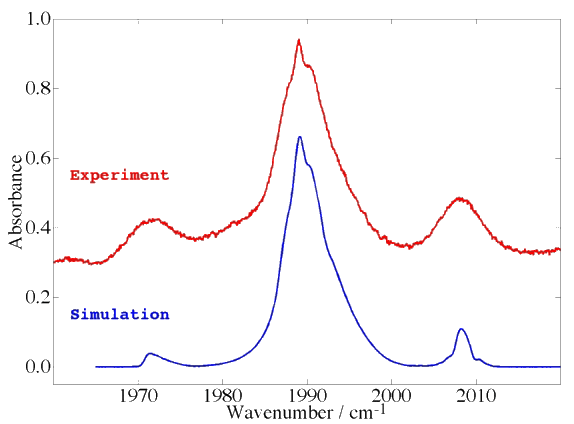 Spectrum from P. Asselin, L. Manceron and P. Soulard / LADIR, Paris, France. Simulation calculated by Michael Rey.[/caption]
Spectrum from P. Asselin, L. Manceron and P. Soulard / LADIR, Paris, France. Simulation calculated by Michael Rey.[/caption]
Reference:
{/slide}
[/kc_column_text][kc_spacing height="30px" _id="630877"][kc_column_text _id="699284"]Gestion et valorisation des données de la recherche
[/kc_column_text][kc_spacing height="20" _id="977070"][kc_column_text _id="704989"]{slide=Plus d'informations}
Le projet "Gestion et Valorisation des Données de la Recherche" a pour but de permettre la description standardisée des ensembles de données issues des recherches scientifiques menées dans les différents laboratoires et équipes de l’OSU THETA, dont fait partie l'équipe SMPCA, d’en favoriser l’accessibilité et de permettre de les valoriser, dans le cadre du mouvement général de l’Open Data.
Il regroupe douze participants issus de 6 structures différentes de Besançon et Dijon :
- OSU THETA,
- Chrono-Environnement,
- UTINAM,
- Biogéosciences,
- Equipe SMPCA du Laboratoire Interdisciplinaire Carnot de Bourgogne,
- Service commun de la documentation de l’Université de Franche-Comté,
Ces participants ont fait le point sur l’avancée du projet le 24 septembre 2015, au terme du travail de 4 mois d’une documentaliste embauchée dans le cadre du Soutien à la Recherche de l’OSU.
Un modèle des métadonnées, compatible avec les modèles existants, a été réalisé, de façon à pouvoir s’appliquer à tous les ensembles de données, quelle que soit la discipline scientifique en jeu. Dans le cadre de la première phase du projet, 9 ensembles de données ont été décrits, dans les domaines de l’hydrogéologie, de l’astronomie, de la physique moléculaire et de l’écologie. Pour l'équipe SMPCA, ceci concerne tout particulièrement les bases de donées de raies spectrales moléculaires calculées MeCaSDa, SHeCaSDa et TFMeCaSDa, dévoloppées dans le cadre du portail européen VAMDC.
La deuxième phase du projet est maintenant la mise au point d’un outil de saisie et de consultation des informations relatives aux données. Cette application devra en outre permettre l’interopérabilité du référencement local avec les systèmes nationaux et internationaux de publication des métadonnées ; elle verra le jour courant 2015.
{/slide}
[/kc_column_text][kc_spacing height="20" _id="321448"][/kc_column][kc_column width="2%" video_mute="no" _id="645623"][/kc_column][/kc_row][kc_row use_container="yes" _id="720429" cols_gap="{`kc-css`:{}}" force="__empty__" animate="fadeInUp||"][kc_column width="12/12" video_mute="no" _id="208815"][kc_spacing height="50px" _id="143651"][/kc_column][/kc_row][kc_row use_container="yes" _id="571764"][kc_column width="20%" _id="770829"][/kc_column][kc_column width="60%" _id="907694"][kc_call_to_action layout="2" title="Accéder aux programmes et banques de données spectroscopiques" desc="__empty__" button_show="yes" button_text="En savoir plus" _id="273657" button_link="https://icb.u-bourgogne.fr/casda-soft/||" css_custom="{`kc-css`:{`any`:{`button`:{`background-color|.kc-cta-button a`:`#ffffff`}}}}"][/kc_column][kc_column width="20%" _id="215508"][/kc_column][/kc_row]
