As a postdoctoral researcher at ICB, I am collaborating with the SIOM team under the guidance of Dr. Celine Dupont and Dr. Bruno Domenichini in partnership with the team at CEA Saclay. My research interests center on multiscale modeling of catalysis, electrolysis for water, and the reduction of carbon dioxide. Specifically, I am currently utilizing molecular modeling techniques to study thin-layer iron-oxide surfaces grown on a platinum substrate for their potential use in water-splitting applications. I earned my Ph.D. in chemistry from ENS de Lyon and IFPEN-Solaize in 2021, under the supervision of Pascal Raybaud and Stephan Steinmann, with a thesis titled « Molecular modeling of the genesis of the active phase of a supported catalyst.”
One of my key objectives as a researcher is to develop a machine learning or an alternative approach to reduce the computational cost of predicting the doping effect on thin-layer iron-oxide surfaces, and to understand the impact of applied external potentials, including the effect of solvent on these systems. This approach can help us to understand how these materials work in real-life situations and how to optimize their performance with lower computational cost.
Ab-initio modeling of doped maghemite
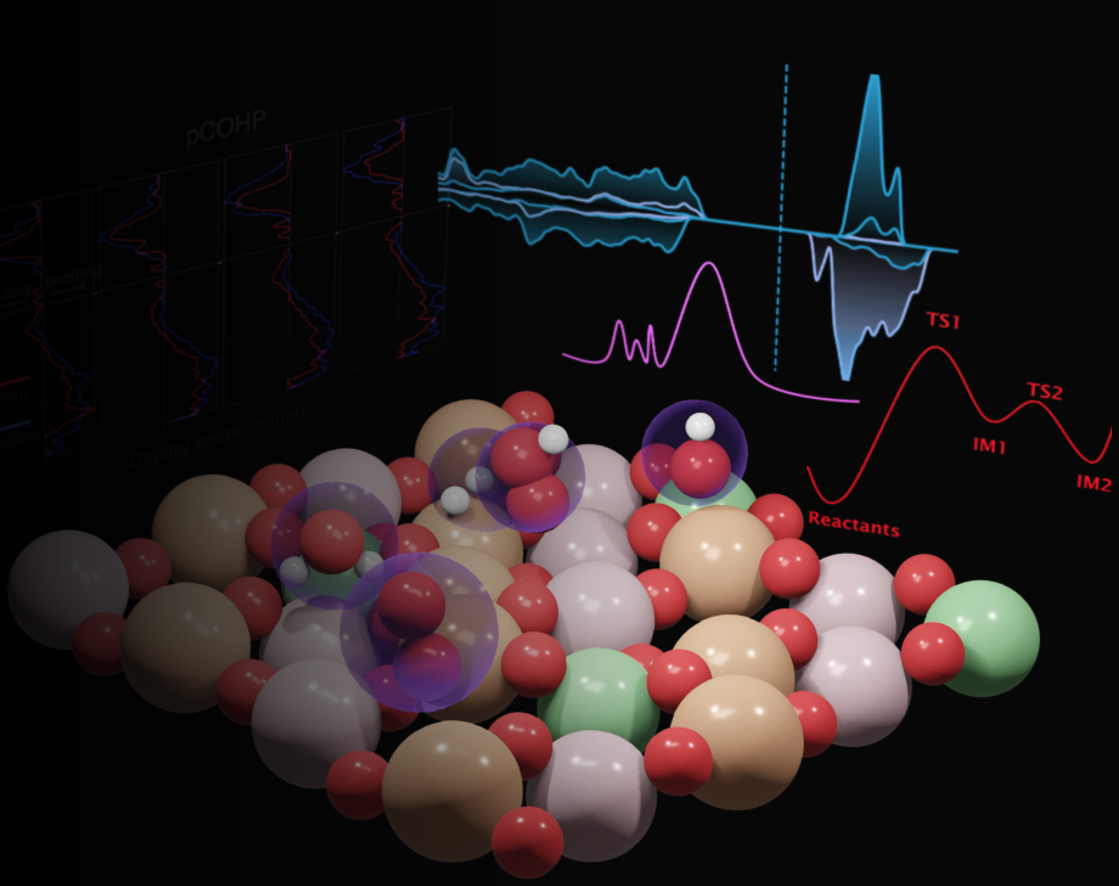
We are working on iron oxides, more specifically on maghemite’s surface terminations bare and supported on Pt-substrate. This study aims to understand the stable surface of maghemite and its reactivity towards HER. Identifying the reactive sites and mechanism of HER would be the next step.
In this study, we examined different maghemite surface terminations when cut along the (001) and (111) directions. We used Hubbard U corrections to localize the d-orbitals of Fe atoms. Our goal was to determine which terminations are thermodynamically stable. We also considered the impact of strain on these terminations. Additionally, we examined the interaction between these terminations and a Pt substrate. Finally, we analyzed various electronic properties to understand the stability and behavior of these surfaces.
We observed that the stability of the (001) termination on a surface with an octahedral vacancy is lower than that of a surface without a vacancy. The stability of the surface also depends on the inner layers, not only limited to the outer layers. A strong reconstruction occurs due to strain, stabilizing some surfaces (AC1, AC2) and destabilizing others (AB, AC3). This reconstruction caused by strain can alter the order of stability and even allow for the inversion of some octahedral-tetrahedral Fe atoms. In general, a larger number of Pt-O interactions are preferred on the Pt substrate. However, despite having the largest interaction energy due to multiple Pt-O interactions, the surface AC3 exhibits lower stability on Pt than AC1 due to major reconstruction induced by strain.
The top layer of the (001)-maghemite surface that has been epitaxially grown on a Pt(001) substrate will be a combination of Fe$_{Th}$ and Fe$_{Oh}$ without vacancy. AD1 surface is the most stable and is not affected by strain in terms of stability among various (111) cases. No significant reconstruction occurs on (111) surfaces due to relaxation or even strain. The combination of the Fe$_{Oh}$ and oxygen termination surface, which is less stable than the others, becomes the most stable on a Pt substrate due to a large number of Pt-O interactions, therefore, contributing to its stability. Overall, the (001) surface is more stable as a bare surface, even when considering the effect of the strain. However, on the Pt substrate, the EF surface of the (111) surface becomes the most stable due to the accumulation of a large amount of interaction energy from multiple Pt-O bonds. As a result, a Fe-octahedral termination can be seen as the most stable surface on Pt(001) when viewed from the top. This observation is in accordance with experiments confirmed by the team at Synchrotron Soleil.

 We are working on iron oxides, more specifically on maghemite's surface terminations bare and supported on Pt-substrate. This study aims to understand the stable surface of maghemite and its reactivity towards HER. Identifying the reactive sites and mechanism of HER would be the next step.
In this study, we examined different maghemite surface terminations when cut along the (001) and (111) directions. We used Hubbard U corrections to localize the d-orbitals of Fe atoms. Our goal was to determine which terminations are thermodynamically stable. We also considered the impact of strain on these terminations. Additionally, we examined the interaction between these terminations and a Pt substrate. Finally, we analyzed various electronic properties to understand the stability and behavior of these surfaces.
We observed that the stability of the (001) termination on a surface with an octahedral vacancy is lower than that of a surface without a vacancy. The stability of the surface also depends on the inner layers, not only limited to the outer layers. A strong reconstruction occurs due to strain, stabilizing some surfaces (AC1, AC2) and destabilizing others (AB, AC3). This reconstruction caused by strain can alter the order of stability and even allow for the inversion of some octahedral-tetrahedral Fe atoms. In general, a larger number of Pt-O interactions are preferred on the Pt substrate. However, despite having the largest interaction energy due to multiple Pt-O interactions, the surface AC3 exhibits lower stability on Pt than AC1 due to major reconstruction induced by strain.
The top layer of the (001)-maghemite surface that has been epitaxially grown on a Pt(001) substrate will be a combination of Fe$_{Th}$ and Fe$_{Oh}$ without vacancy. AD1 surface is the most stable and is not affected by strain in terms of stability among various (111) cases. No significant reconstruction occurs on (111) surfaces due to relaxation or even strain. The combination of the Fe$_{Oh}$ and oxygen termination surface, which is less stable than the others, becomes the most stable on a Pt substrate due to a large number of Pt-O interactions, therefore, contributing to its stability. Overall, the (001) surface is more stable as a bare surface, even when considering the effect of the strain. However, on the Pt substrate, the EF surface of the (111) surface becomes the most stable due to the accumulation of a large amount of interaction energy from multiple Pt-O bonds. As a result, a Fe-octahedral termination can be seen as the most stable surface on Pt(001) when viewed from the top. This observation is in accordance with experiments confirmed by the team at Synchrotron Soleil.
We are working on iron oxides, more specifically on maghemite's surface terminations bare and supported on Pt-substrate. This study aims to understand the stable surface of maghemite and its reactivity towards HER. Identifying the reactive sites and mechanism of HER would be the next step.
In this study, we examined different maghemite surface terminations when cut along the (001) and (111) directions. We used Hubbard U corrections to localize the d-orbitals of Fe atoms. Our goal was to determine which terminations are thermodynamically stable. We also considered the impact of strain on these terminations. Additionally, we examined the interaction between these terminations and a Pt substrate. Finally, we analyzed various electronic properties to understand the stability and behavior of these surfaces.
We observed that the stability of the (001) termination on a surface with an octahedral vacancy is lower than that of a surface without a vacancy. The stability of the surface also depends on the inner layers, not only limited to the outer layers. A strong reconstruction occurs due to strain, stabilizing some surfaces (AC1, AC2) and destabilizing others (AB, AC3). This reconstruction caused by strain can alter the order of stability and even allow for the inversion of some octahedral-tetrahedral Fe atoms. In general, a larger number of Pt-O interactions are preferred on the Pt substrate. However, despite having the largest interaction energy due to multiple Pt-O interactions, the surface AC3 exhibits lower stability on Pt than AC1 due to major reconstruction induced by strain.
The top layer of the (001)-maghemite surface that has been epitaxially grown on a Pt(001) substrate will be a combination of Fe$_{Th}$ and Fe$_{Oh}$ without vacancy. AD1 surface is the most stable and is not affected by strain in terms of stability among various (111) cases. No significant reconstruction occurs on (111) surfaces due to relaxation or even strain. The combination of the Fe$_{Oh}$ and oxygen termination surface, which is less stable than the others, becomes the most stable on a Pt substrate due to a large number of Pt-O interactions, therefore, contributing to its stability. Overall, the (001) surface is more stable as a bare surface, even when considering the effect of the strain. However, on the Pt substrate, the EF surface of the (111) surface becomes the most stable due to the accumulation of a large amount of interaction energy from multiple Pt-O bonds. As a result, a Fe-octahedral termination can be seen as the most stable surface on Pt(001) when viewed from the top. This observation is in accordance with experiments confirmed by the team at Synchrotron Soleil.